We are GxP & regulatory compliance specialists helping therapeutic goods manufacturers, testing laboratories
and biotechnology companies
WHAT WE DO
We are GxP consultants, specializing in helping pharmaceutical and medical device manufacturers and sponsors, testing laboratories and associated supporting companies gain or maintain their GMP manufacturing license or ISO accreditation, and everything in-between!
QSN also helps emerging life-science startups bridge the gap between R&D and commercial operations by guiding them through the transition to GMP sponsorship or greenfield manufacturing. We provide practical, phase-appropriate support for GLP, GCP and GMP. We will help you with system, process, product, documentation, and validation strategies that meet regulatory expectations without over-engineering — enabling you to scale confidently, achieve compliance, and bring your innovation to market.
Check out our services - we can help you with:
General GxP requirements - GLP, GCP and GMP
Quality Management Systems (QMS) / Pharmaceutical Quality Systems (PQS)
Validation
Laboratory systems, equipment, methods, specifications etc.
Product development guidance and preparation for commercialization
Quality Assurance
Training
Audit readiness
Third party or internal audits
Electronic system implementation
Regulatory compliance
Production & facilities
Compliance to Good Distribution Practices (GDP) and supply chain.
WHY WORK WITH US
We often have clients who return to QSN multiple times because of the great experience they have working with us.
QSN consultants are knowledgeable in their niche areas of expertise. They have been in the industry for many years, working with clients, so they have authority and know what works in practice.
We also pride ourselves on being flexible and agile, able to quickly engage and assist clients, wherever they are on their compliance journey.
And we won't over-complicate things, we keep it simple!

OUR FRAMEWORK
We work with all clients using our QSN framework. Whether you need help with all stages or just one or two to meet your goals, this is our roadmap for delivering your project.
Check out our QSN framework below.
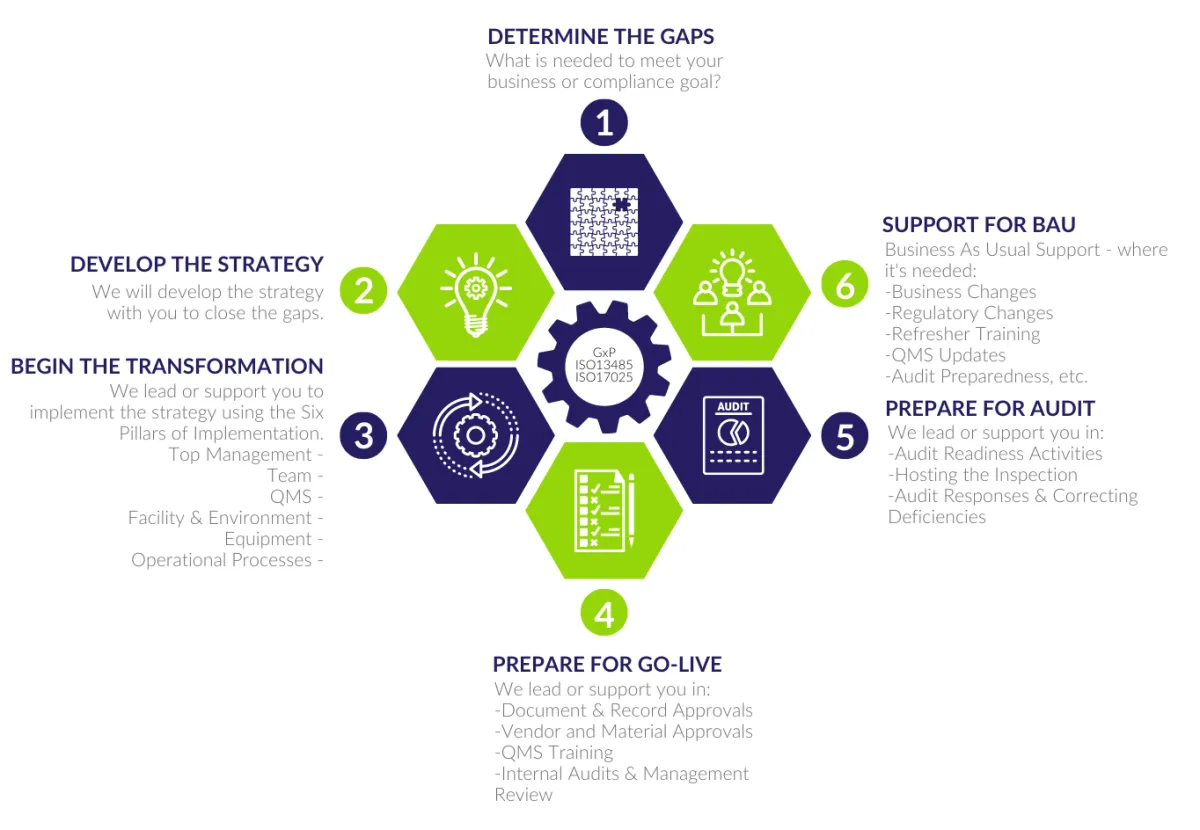
SIX PILLARS OF IMPLEMENTATION
As part of the framework Stage 3 - Begin the Transformation, QSN uses the following compliance pillars to help you implement your project.
So, whether you have a new greenfield site, or a documentation project, we understand the wider view and can help you deliver a simple, compliance solution.

SOME OF THE INDUSTRIES WE SUPPORT

PHARMACEUTICAL

MEDICAL DEVICES
BIOTECH
TRANSFORM YOUR PROCEDURES INTO
USER-FRIENDLY DOCUMENTS USING PLAIN LANGUAGE







