SITE LICENSING AND ACCREDITATION
We help companies wanting to become GMP licensed and ISO accredited.
We routinely support accreditation to the following:
Good Manufacturing Practice (GMP) - pharmaceuticals or veterinary facilities
ISO13485 - Medical device manufacturing, including IVDs and software
ISO17025 - Testing and calibration laboratories, particularly those supporting clinical trials or biotech
ISO9001 - Quality Management Systems (medtech, biotech etc.)
We will help you understand your compliance and accreditation responsibilities and any business risks, as well as realistic timelines and expenditure, associated with your project.
We can provide day-to-day direction or be another set of hands, so your team owns the transition and increases their knowledge in the accreditation standard requirements.
We use the QSN Framework and the Six Pillars of Implementation to help you across all areas of development and changes on your site.
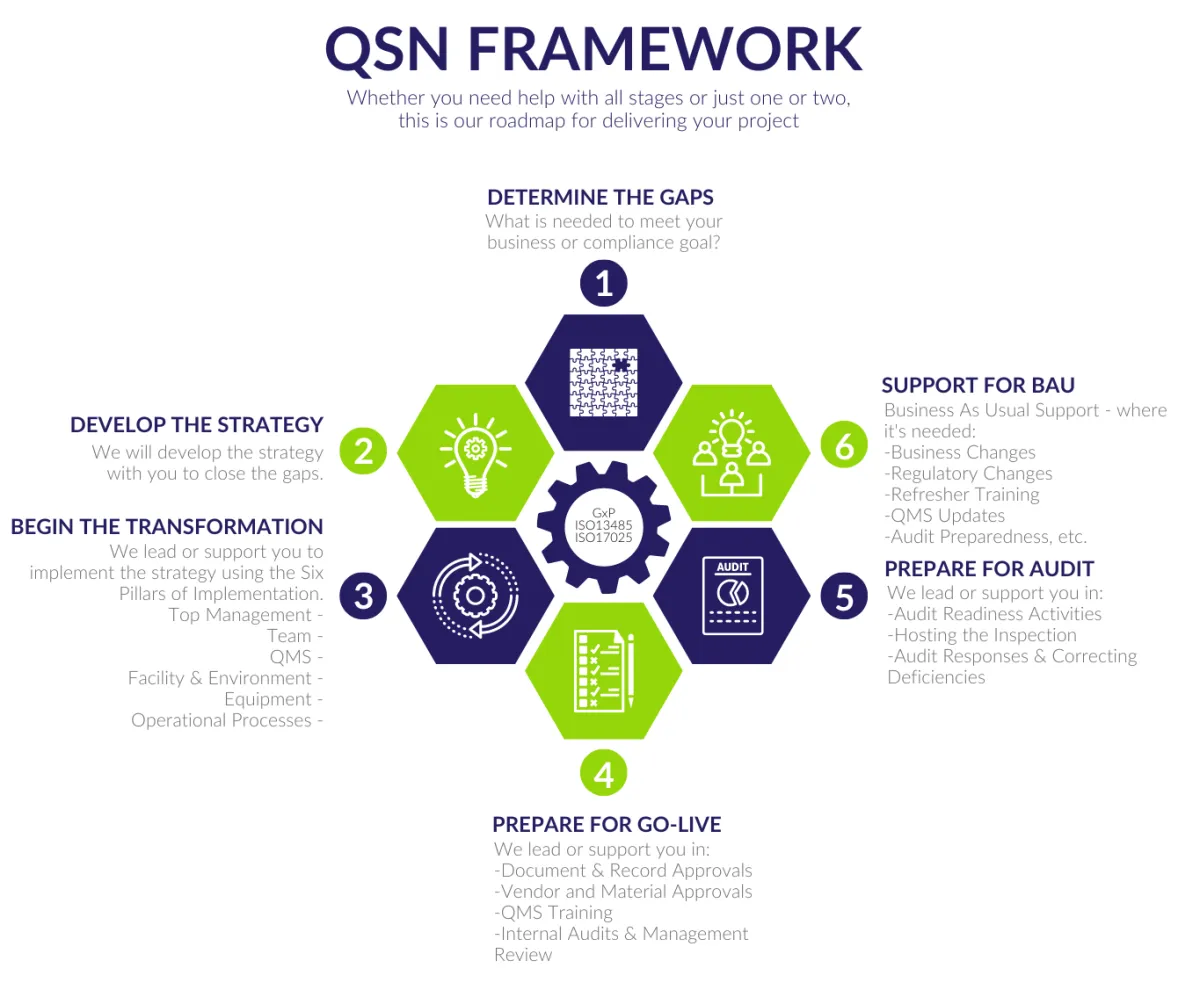
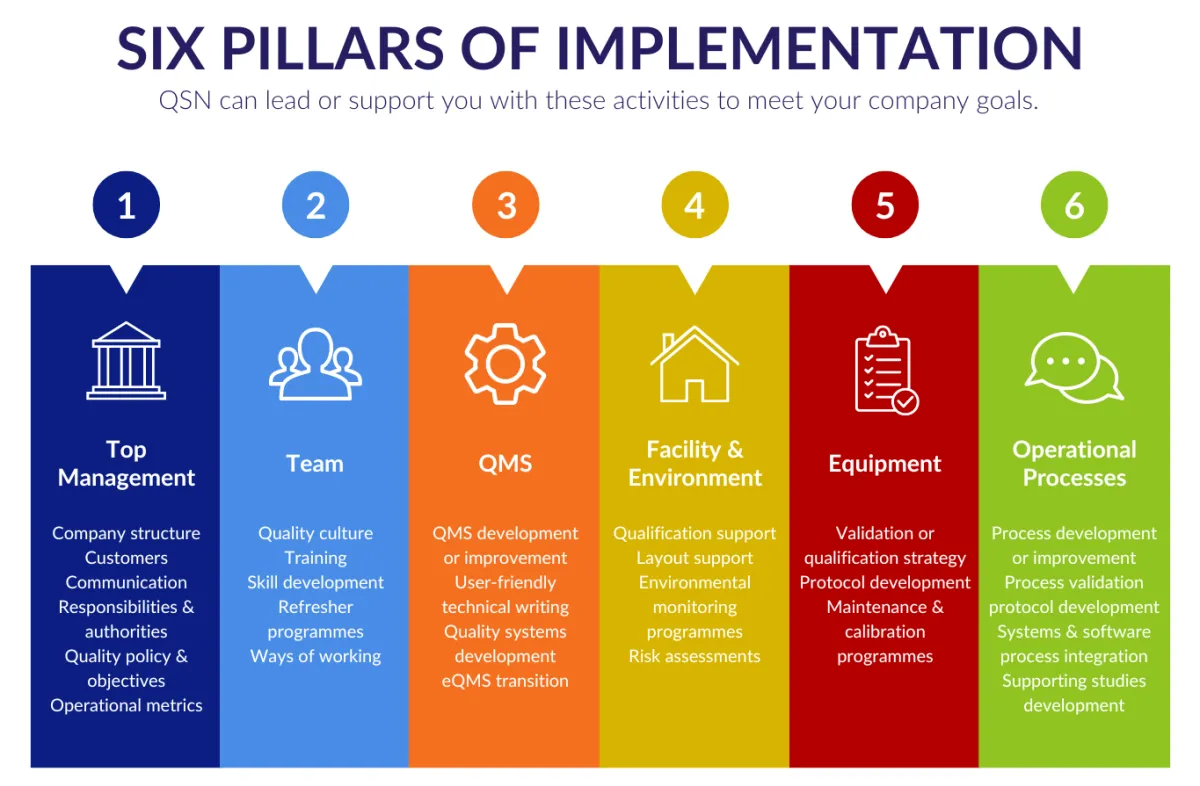
Although we are not Regulatory Affairs specialists, we can assist you in also connecting with experts in product registration.
