PRODUCT DEVELOPMENT COMPLIANCE
Helping R&D Teams Transition Towards Commercialization with Confidence
Emerging R&D or startup companies often struggle to balance innovation with increasing compliance rigor. Laboratory methods, lab-scale batches and informal practices that worked for early development often do not transition well to clinical trial and commercial manufacturing.
Without a phase-appropriate Pharmaceutical Quality System (PQS) – GLP, GCP, GMP - the risk is high—overbuilding systems too early or underpreparing for manufacturing, validation, and licensing requirements.
The same is true for design and development of medical devices in ISO13485, where companies need a robust Quality Management System (QMS) that is suited to design stages - prototype, product verification, clinical validation and transfer to commercial manufacturing.
At QSN we help you bridge that gap — turning scientific success into regulatory compliance success.
We guide you to establish strong compliance practices and rigor appropriate for each stage of product development using our proven QSN R&D Framework and the Six Pillars of Implementation for R&D.
The Challenges We Solve
Inadequate phase-appropriate compliance – Early-stage systems don’t scale for GxP expectations.
Limited understanding of GxP and sponsor responsibilities – Even when testing or manufacture is outsourced, the sponsor remains accountable.
Unverified processes and data integrity risks – Informal methods and incomplete validation threaten product quality and submission timelines.
Disconnected operations – Data, documentation, and process control systems evolve separately, creating inefficiencies and compliance gaps.
Our R&D Framework
Our structured, end-to-end R&D Framework below, ensures your product, compliance, and operational systems start to work together to achieve your clinical or phase-appropriate goals.
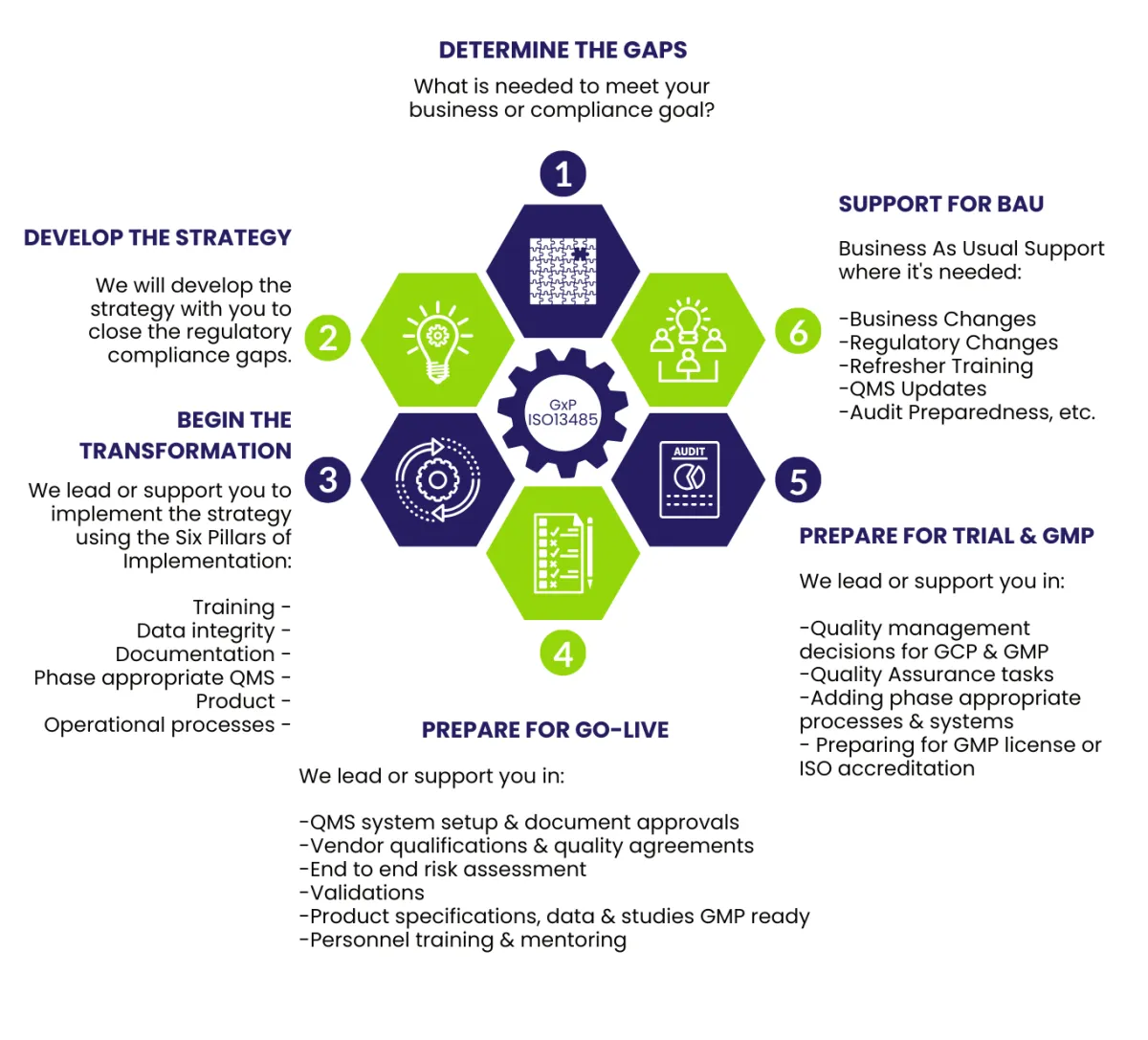
The Six Pillars of Implementation
Our Six Pillars Framework ensures all parts of your early-stage compliance ecosystem work together to deliver robust, scalable, and phase-appropriate systems for your R&D team.
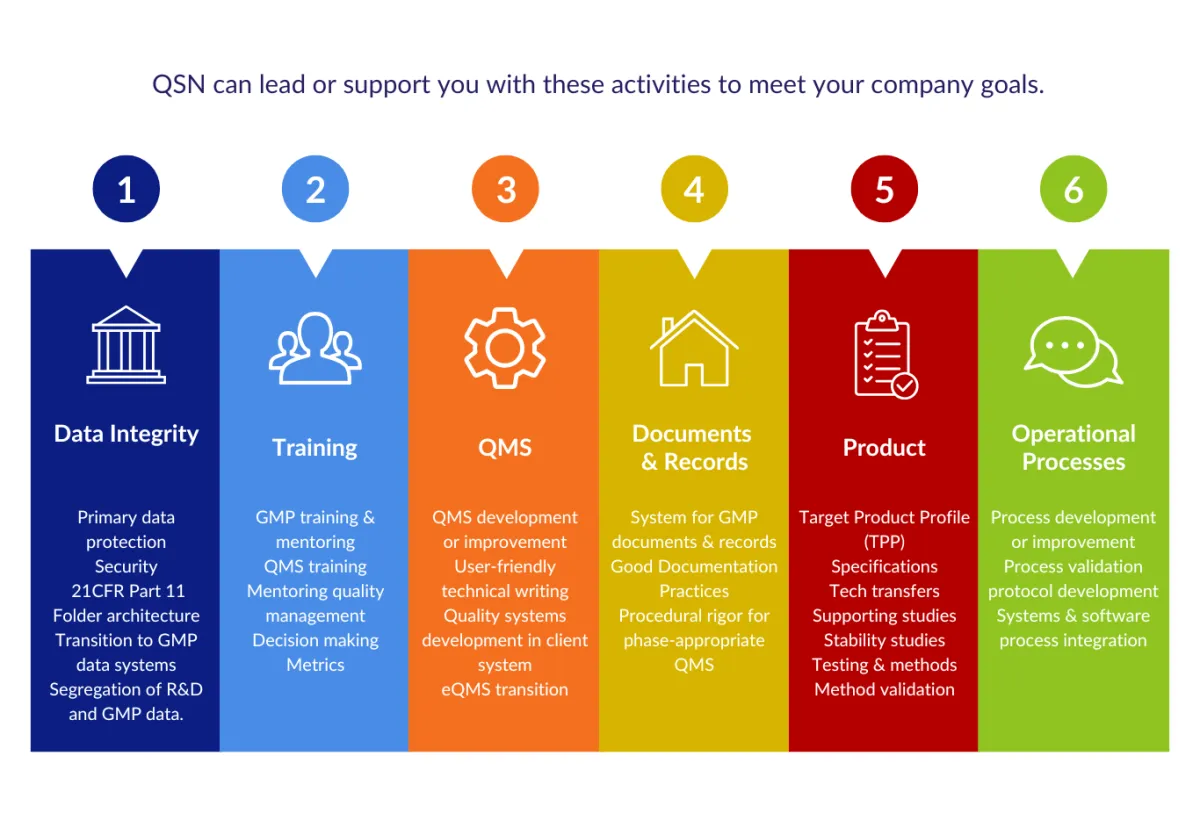
Ready to Commercialize with Confidence?
Whether you’re scaling for clinical manufacture, preparing for GMP licensing, or transitioning to ISO 13485 accreditation — QSN helps you connect the dots between R&D, quality, and business performance. Book a Discovery Call to assess your readiness and receive a tailored commercialization roadmap.
