LATEST NEWS
Does your training system promote learning and process improvement?
How many companies have you started a new job at, and their way to train you in your job was almost exclusively to read a list of procedures?
Did it work? Or did you learn more by doing rather than reading?
Pharma and medical device companies are expected to have detailed procedures as part of their compliance requirements.
Many have assessments and more hands-on training methods, especially for shop floor employees, but many just roll out the SOP list. Here, read these and sign the form! And we wonder why mistakes happen and employees are disengaged?
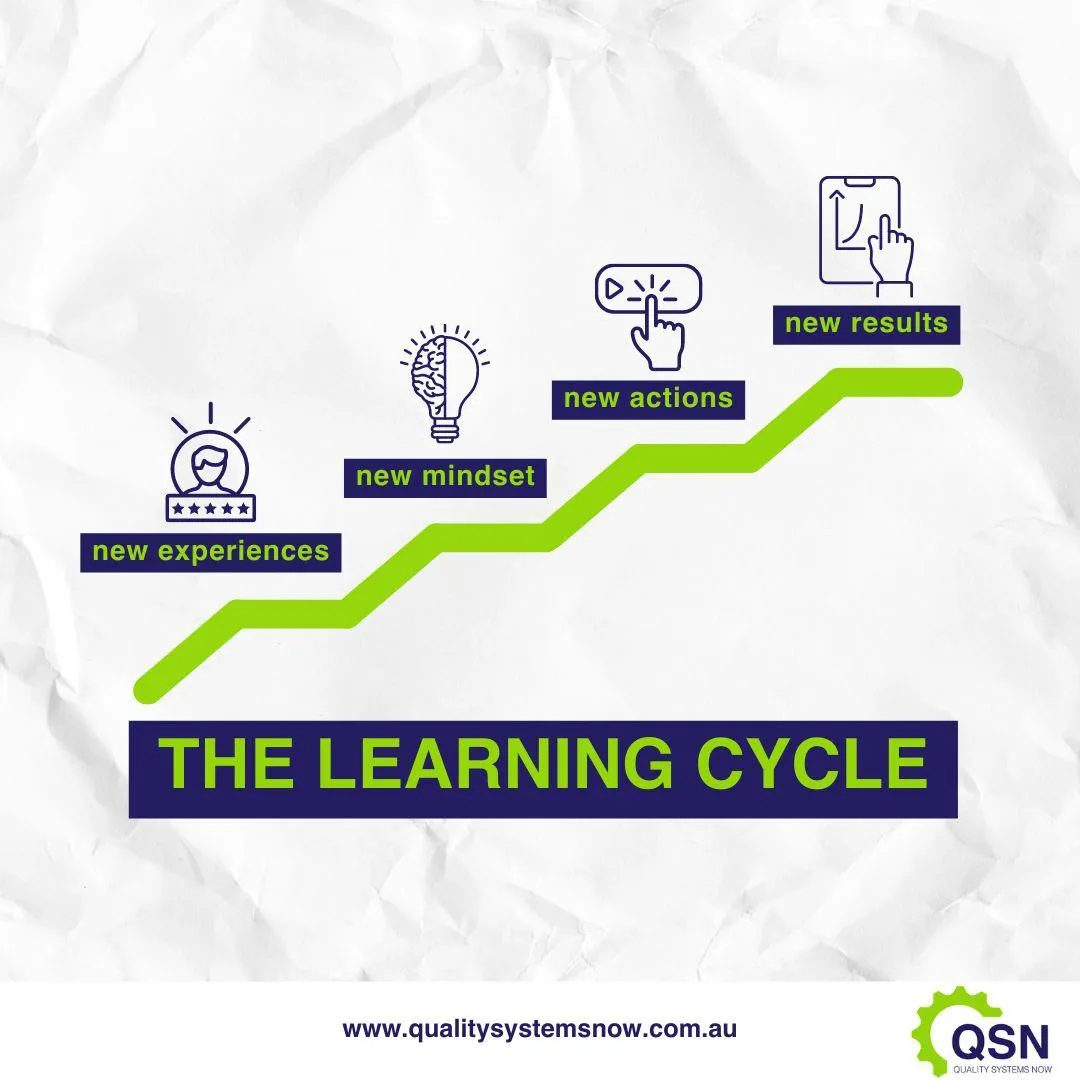
The intent of ICH Q10, Quality Management Maturity (QMM) and quality culture, is to ensure competence and compliant behaviours. What could we do differently in our training programs that aligns with these 3 - especially quality culture? Because experience-based learning changes behaviours and therefore the end result.
When employees, either to learn their job, or to develop process improvements, are exposed to new learning experiences, then new mindsets are created. When we expand our thinking, then actions follow. And when there are new approaches to situations, new results also follow.
Engaging continuously in this type of learning cultivates a culture of continuous improvement in quality, where employees use what they know in order to improve what they do. What's one thing you would like to see improved in your company learning approach towards a stronger quality culture? Put it in the chat, I'd love to hear from you! And don't forget to check out the Mature QMS Mastermind group
